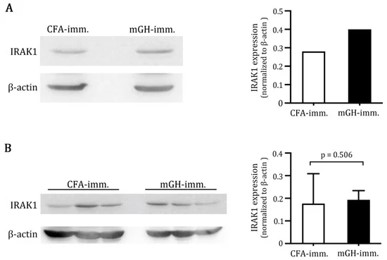
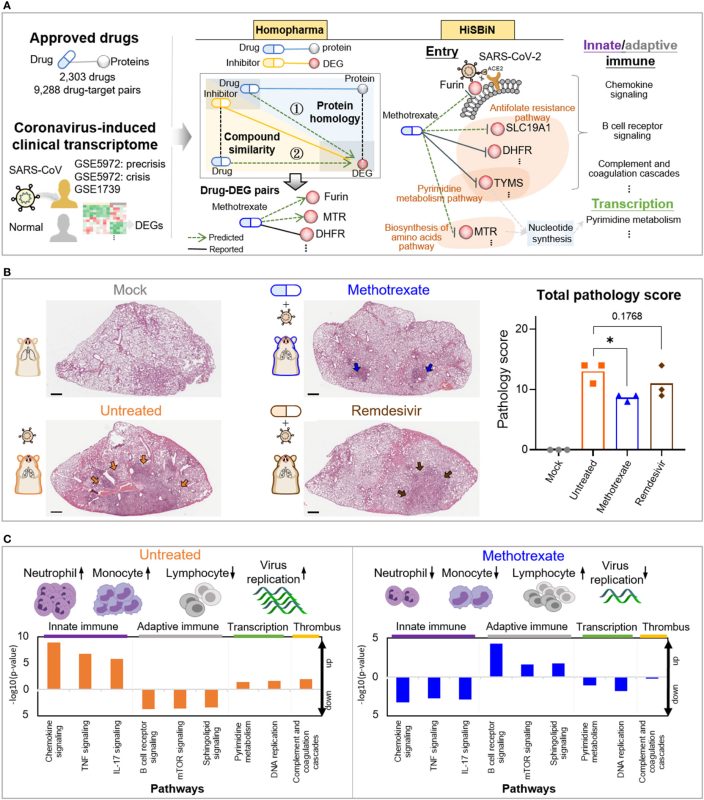
鄒協成副教授研究團隊發表研究成果於 Inflammation and Regeneration
連結網址:https://pubmed.ncbi.nlm.nih.gov/36797799/
Abstract
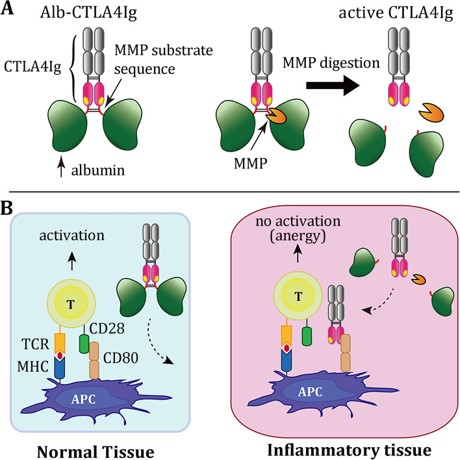
Background: CTLA4Ig is a dimeric fusion protein of the extracellular domain of cytotoxic T-lymphocyte protein 4 (CTLA4) and an Fc (Ig) fragment of human IgG1 that is approved for treating rheumatoid arthritis. However, CTLA4Ig may induce adverse effects. Developing a lesion-selective variant of CTLA4Ig may improve safety while maintaining the efficacy of the treatment.
Methods: We linked albumin to the N-terminus of CTLA4Ig (termed Alb-CTLA4Ig) via a substrate sequence of matrix metalloproteinase (MMP). The binding activities and the biological activities of Alb-CTLA4Ig before and after MMP digestion were analyzed by a cell-based ELISA and an in vitro Jurkat T cell activation assay. The efficacy and safety of Alb-CTLA4Ig in treating joint inflammation were tested in mouse collagen-induced arthritis.
Results: Alb-CTLA4Ig is stable and inactive under physiological conditions but can be fully activated by MMPs. The binding activity of nondigested Alb-CTLA4Ig was at least 10,000-fold weaker than that of MMP-digested Alb-CTLA4Ig. Nondigested Alb-CTLA4Ig was unable to inhibit Jurkat T cell activation, whereas MMP-digested Alb-CTLA4Ig was as potent as conventional CTLA4Ig in inhibiting the T cells. Alb-CTLA4Ig was converted to CTLA4Ig in the inflamed joints to treat mouse collagen-induced arthritis, showing similar efficacy to that of conventional CTLA4Ig. In contrast to conventional CTLA4Ig, Alb-CTLA4Ig did not inhibit the antimicrobial responses in the spleens of the treated mice.
Conclusions: Our study indicates that Alb-CTLA4Ig can be activated by MMPs to suppress tissue inflammation in situ. Thus, Alb-CTLA4Ig is a safe and effective treatment for collagen-induced arthritis in mice.